For over 40 years, Butterworth Laboratories has provided independent, contract analytical services to the global pharmaceutical and related industries.
Are Ambient Temperatures in the UK Becoming a Risk Factor?
23 June 2023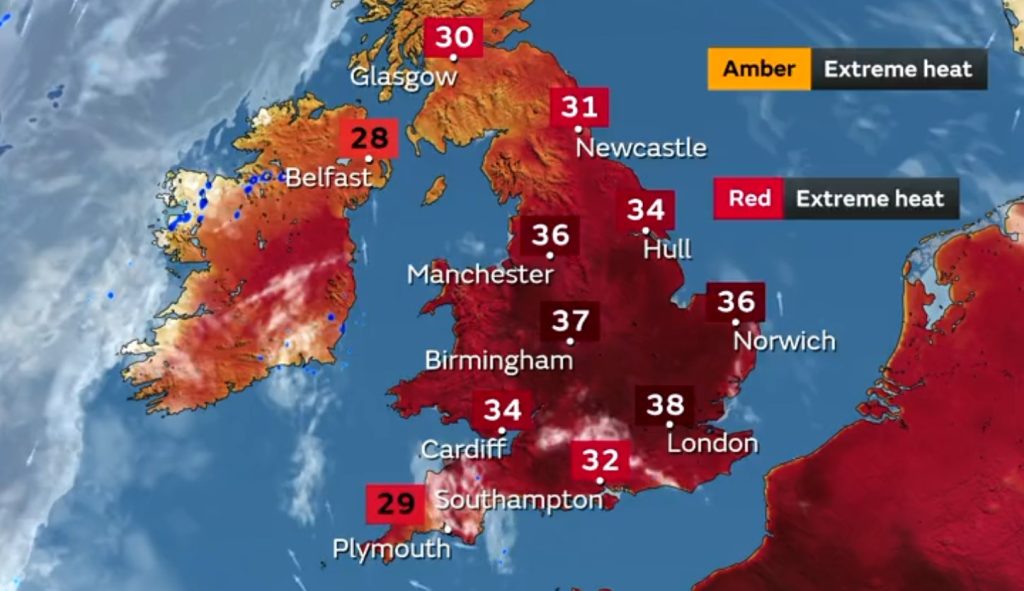
Ambient temperatures in the UK are not something we usually associate as a potential risk factor. But as a weather map from the summer of 2022 illustrates, is this something we should be taking more notice of?
Butterworth is used to receiving samples from clients in packaging containing temperature monitors for samples that must be kept refrigerated or frozen. However, we have yet to receive the same care taken with samples to be stored at ambient temperatures. We maintain and monitor our sample stores at 15 – 25°C, meeting the requirements of Pharmacopoeias. However, we were aware during last summer, that for samples in transit, temperatures may well have been over 25°C upper limit. If so, could the samples have been affected?
Are clients submitting samples considering this as a risk factor, when sending small batches of samples for testing? Have the regulators considered some sort of advice on this subject? Should suppliers of reference materials, be taking note of this risk when supplying materials to be stored at ambient temperatures?